- TOP
- Stories
- Survey Findings on Compliance by Pharmaceutical Companies and Medical Sales Representatives
- Healthcare Research
- Research Reports
Survey Findings on Compliance by Pharmaceutical Companies and Medical Sales Representatives

A survey conducted in July by Nikkei Research looked into compliance awareness among employees of pharmaceutical companies and asked physicians about pharmaceutical companies' responses concerning compliance. It was revealed that 14% of MRs (medical sales representatives) have ignored the rules at least once in the past.
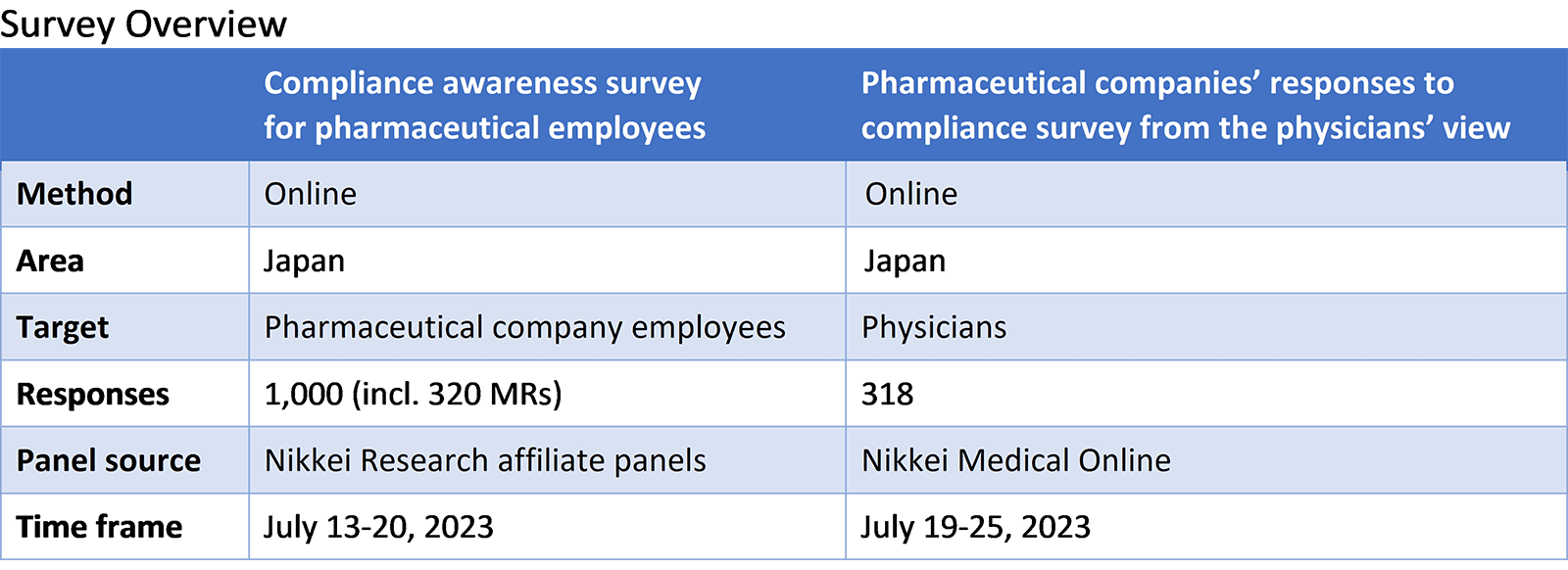
Nikkei Research analyzed the data and compared the results for domestic Japanese pharmaceutical companies with those of foreign companies.
When comparing the rate of general compliance training program implementation, the rate for foreign firms was greater than that for domestic Japanese companies.
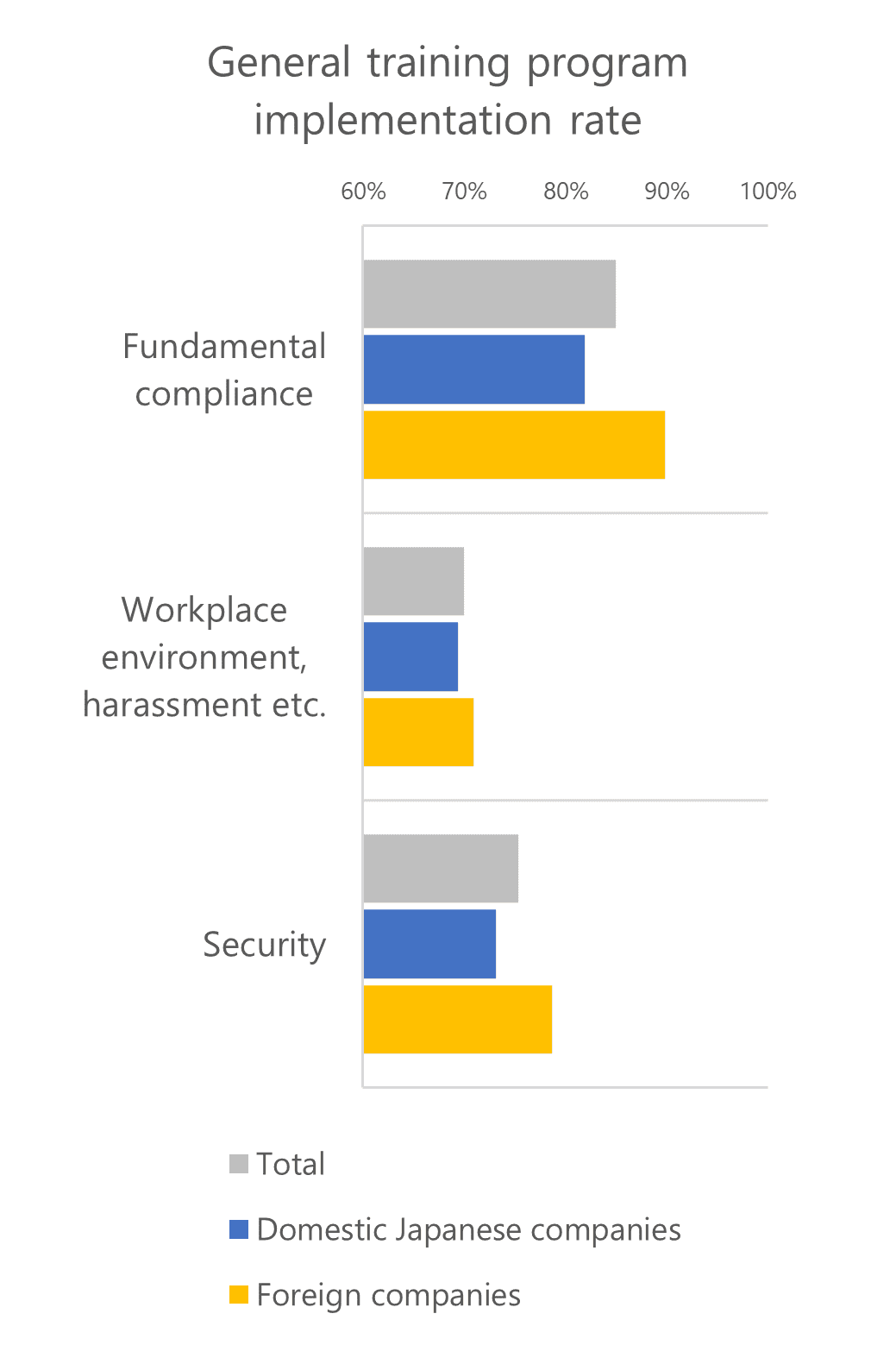
Where there may have been language and terminological differences, foreign firms displayed a higher implementation rate for specialized programs, as well. This was conspicuously true for sales information provision activity guidelines.
For foreign companies, Japanese rules and guidelines relevant in this case are only applicable when such companies conduct sales activities in Japan. Despite this fact, it seems that these companies display a high degree of awareness and make great efforts to be sure to comply.
Reported incidents of inappropriate promotion have been declining, and every pharmaceutical company seems focused on carrying out sales activities aligned with regulations. However, the Ministry of Health, Labour and Welfare of Japan (MHLW) continuously highlighted incidents of falsification, with 23 cases of suspected violation, in its 2022 edition of the industry oversight monitoring reports.
Note that due to factors such as membership, reading the results on compliance with the Japan Pharmaceutical Manufacturers Association (JPMA) code requires caution. However, there was a trend of higher implementation rates regarding the companies' own rules.
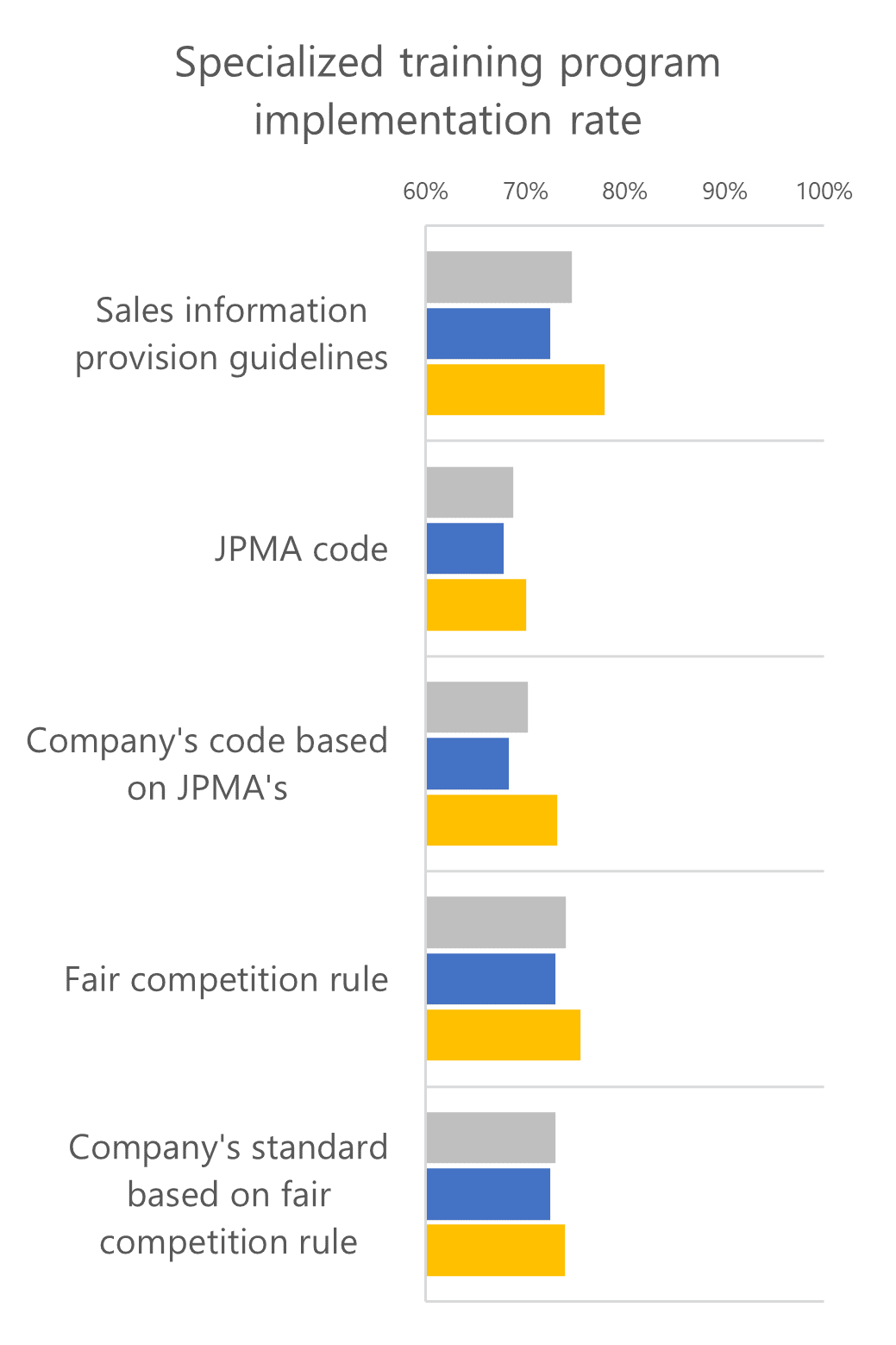
When comparing whether training corresponded to daily operations, 70% of the respondents in foreign companies answered that the training sufficiently matched their actual business operations. This suggests that the training at foreign firms is highly aligned with their businesses, while the content and quality of the training might be slightly different from those at domestic companies.
Further, the survey discovered that MRs and doctors are concerned about the rules that pharmaceutical companies implement on their own. The challenges MRs face during their daily operations, such as when providing information and making tough decisions, sometimes force them to the boundary of what is right and what is wrong. On the other hand, physicians are annoyed by the different rules and policies that each pharmaceutical company has, and at the lack of sufficient explanations, depending on the MRs they encounter.
Other analyses of the survey data were carried out, such as comparing pioneer medicine manufacturers and generic manufacturers.
More details of the survey results are available from the below link.
https://www.nikkei-r.co.jp/english/column/9233
NIKKEI Research

We help to satisfy your research needs and derive meaningful insights. Our overseas offices in Thailand and the United States provide you with support globally. With over 50 years of experience, we specialize in numerous areas across industries and are involved in both BtoC- and BtoB-related endeavors. We cover a variety of topics, from market validation, employee engagement and customer experience to branding, complex analysis and beyond.
See More
